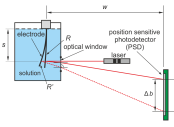
Changes of the interfacial stress with electrode potential in the Ru|0.1 M perchloric acid system
The paper by
M. Ujvári,
S. Vesztergom,
Cs.B. Pénzes and
G.G. Láng
is published in Electrochemistry Communications (2013, vol. 28, pp. 111–113).
Abstract:
Electrochemical and mechanical properties of ruthenium films electrodeposited on gold have been investigated in aqueous perchloric acid solutions. Cyclic voltammograms recorded at slow sweep rates in rotating disk experiments were highly asymmetric with respect to the electrode potential axis, and a negative current could be observed even during the positive sweep. Changes of the interfacial stress were measured by the bending beam method as functions of the electrode potential and of the concentration of chloride ions. The results are consistent with the earlier observation that perchlorate ions can be electrochemically reduced to Cl- on ruthenium. The resulting chloride ions decrease the interfacial stress and exert an inhibiting effect on the reduction process indicating the role of competitive adsorption. The desorption rate of Cl- is significantly influenced by the hydrodynamic conditions, probably through desorption/diffusion coupling.
